+91 9739192366
enquiry@drklinisch.com
Mon - Fri: 9.00am - 11.00pm
Dr. Klinisch Research provide comprehensive Clinical Trial Management Services for Medical Device Industries.
We focus on turning a new leaf in the journey (of the Medical Device companies) by adding scientific values through translational research.




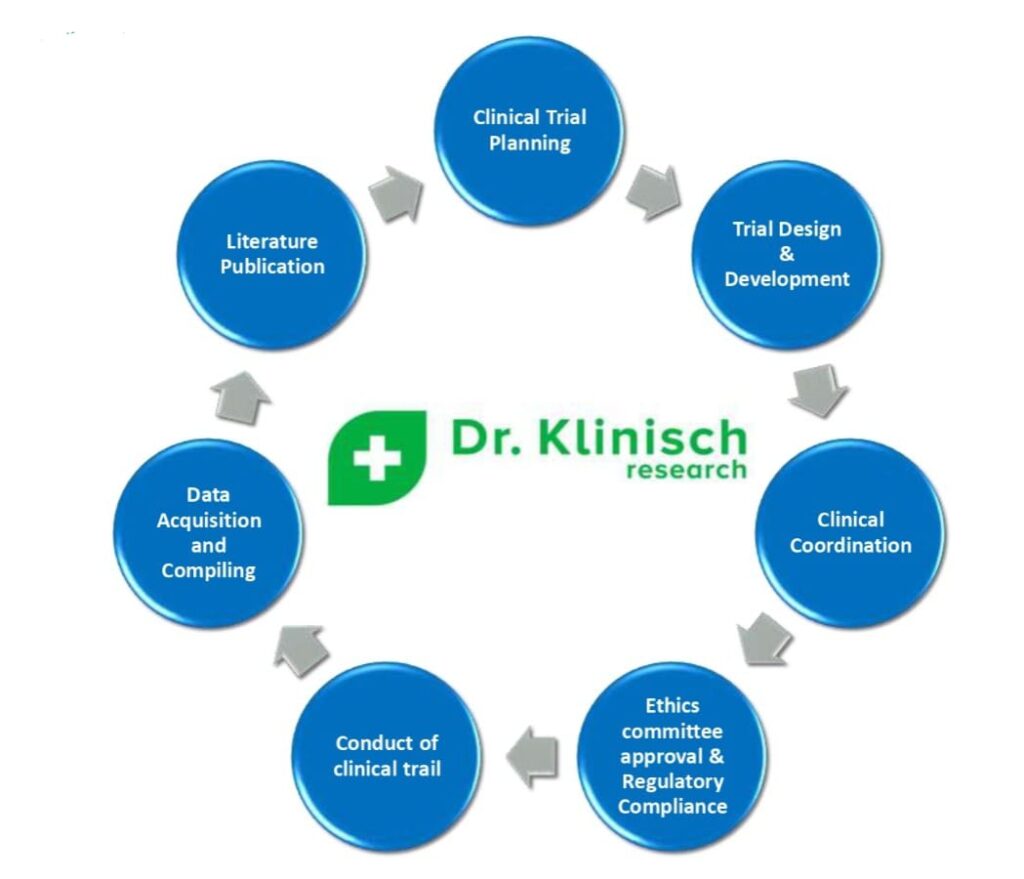




To provide comprehensive, and cost-effective clinical strategies
To provide ethical and time bound services to the client conforming to global regulatory requirements



"Where the world of healthcare meets"
Participated in Arab health 2024 at Dubai World Trade Centre where 3,450 exhibitors from 180 participating countries with 110,000 healthcare professionals had participated.



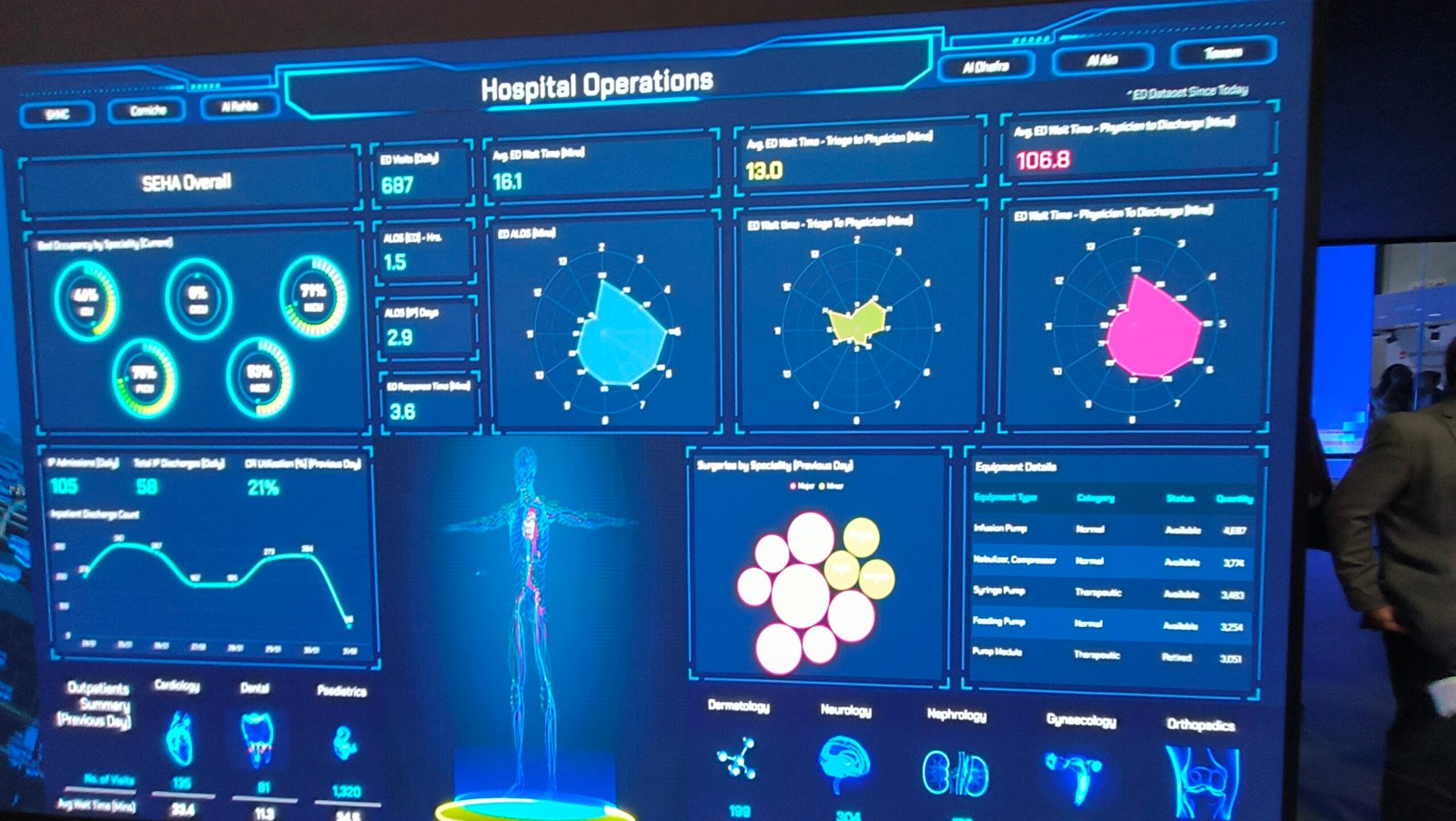
"Where the world of healthcare meets"





India's Largest & No.1 Hospital Equipment Expo Your Gateway To Indian Healthcare






If you find yourself constantly bookmarking health sections on news. Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut

If you find yourself constantly bookmarking health sections on news. Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut

If you find yourself constantly bookmarking health sections on news. Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut

Organized by Medexpert Business Consultants Pvt Ltd, promoted by Dr. Manivannan S, Managing Director of Kauvery group of hospitals a 1200 bedded Hospital group in South India.